Enzyme Simulation
In our Human Body Systems course, students learn about the digestive system and enzymes that help break down various food macromolecules. As a bonus, most of my HBS students are also taking chemistry, so we have opportunity to share ideas across the disciplines.
In the past, we did a hands-on lab measuring the rate of reaction of catalase to break down hydrogen peroxide. We could vary the temperature, pH, agitation, concentration, or a number of other independent variables to determine their effect on the efficacy of the catalase enzyme. While I love doing hands-on labs with students, this one took several days to collect enough data, and the data we collected was often unreliable–students measured reaction rates using gas pressure sensors. Any leaks in the flasks, stoppers, or hoses would give us bad data, and then using the computer interface to find the slope of the pressure increase (our proxy for reaction rate) was a small hurdle as well.
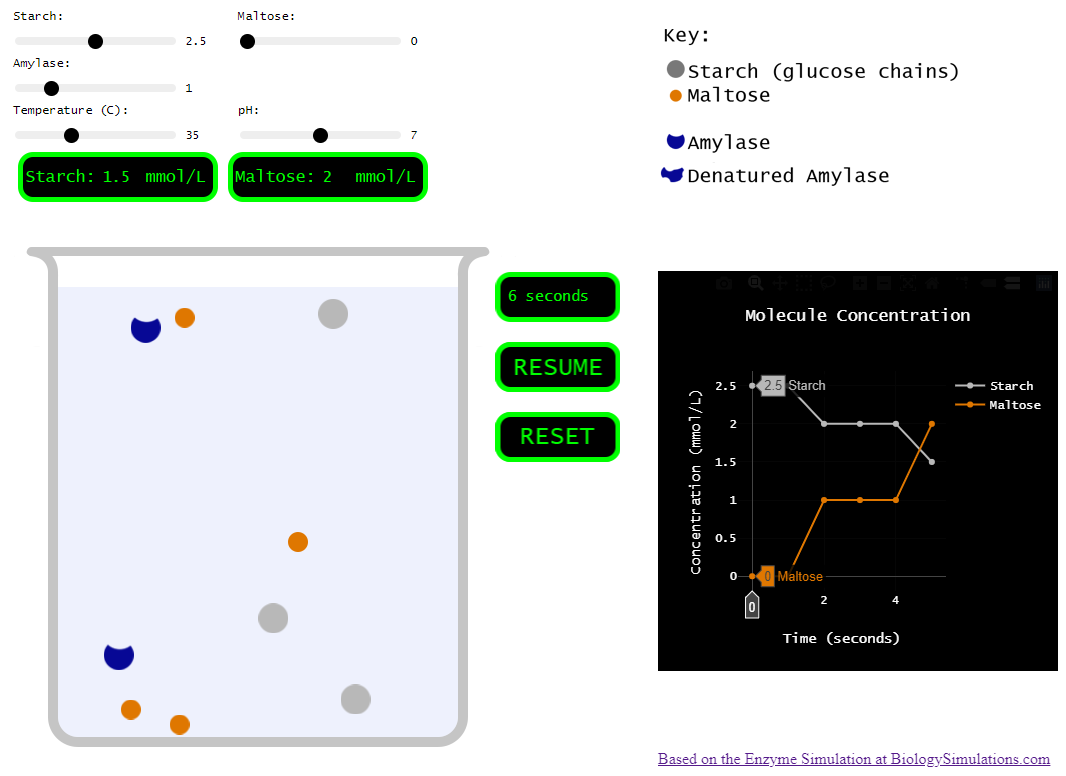
A couple years ago, my class was running behind and I decided to forego the hands-on portion, but I still needed students to learn the effects of various conditions on enzymes. So, I found a great enzyme simulation at Biology Simulations. Very cool! I love how the collisions between molecules are clearly shown and how we can change so many variables to test their effect on the reaction.
However, for my purposes, this simulation incorporated a bit too much. There are multiple enzymes. There are multiple reactants and products. There are inhibitors! SO many things to play around with. Which is all good, but I needed my students to understand the basics. How do things like pH, temperature, and concentration affect an enzyme…
So, I set out to pare the biology simulations version down to just a few variables and a single enzyme.
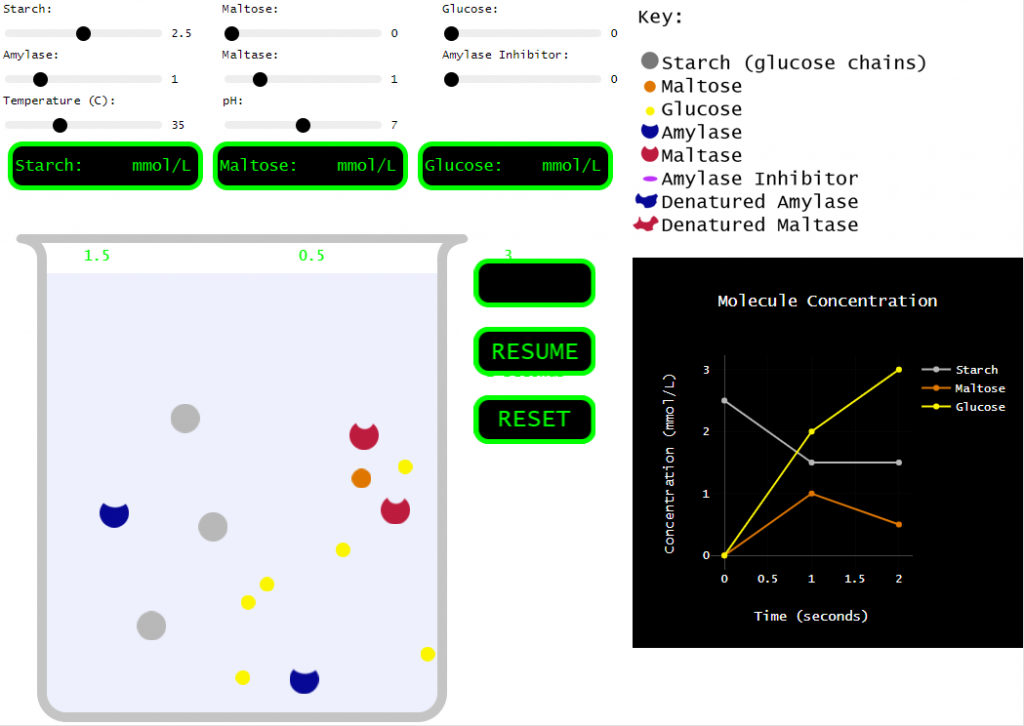
Original (Biology Simulations) version
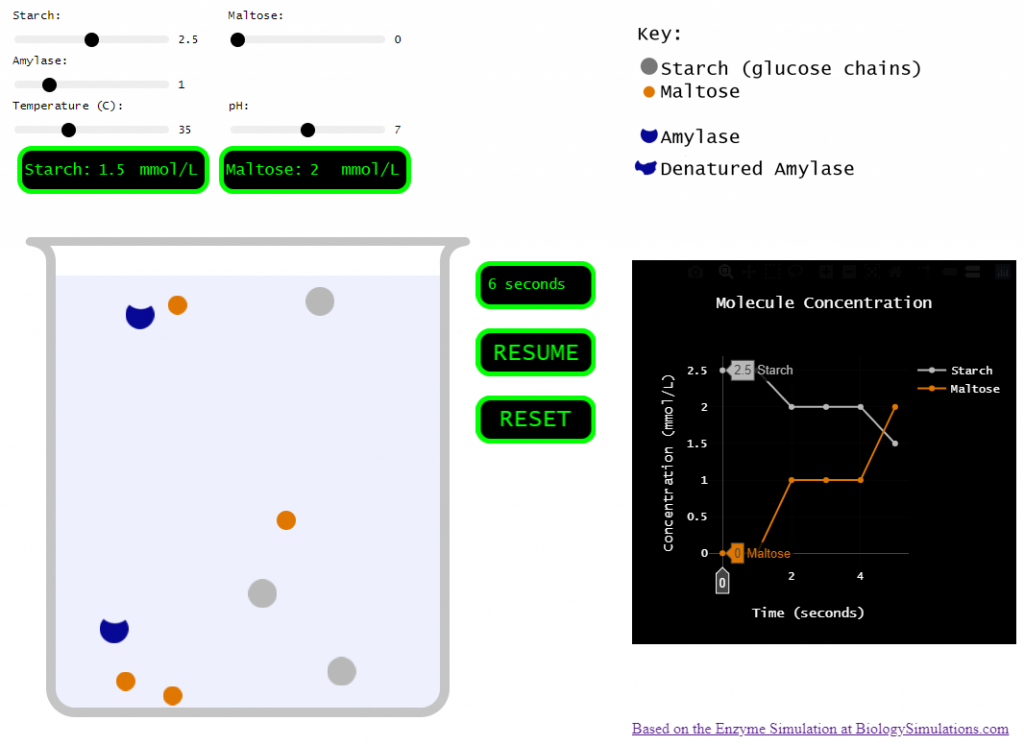
Simplified version
Click the button below to try it out. I also put together a very bare-bones ‘worksheet’ to go along with the simulation. Let me know if this is useful to you.